Molar Mass Of Isopentyl Alcohol
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 3-Methylbutyl acetate | |
| Systematic IUPAC name 3-Methylbutyl ethanoate | |
| Other names Isopentyl acetate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| Beilstein Reference | 1744750 |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.004.240 |
| EC Number |
|
| Gmelin Reference | 101452 |
| KEGG |
|
| PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 1104 1993 |
| CompTox Dashboard (EPA) |
|
| InChI
| |
| SMILES
| |
| Properties | |
| Chemical formula | C 7 H xiv O 2 |
| Molar mass | 130.187 g·mol−1 |
| Appearance | Colorless liquid |
| Scent | Banana-like[one] |
| Density | 0.876 grand/cm3 |
| Melting signal | −78 °C (−108 °F; 195 K) |
| Boiling betoken | 142 °C (288 °F; 415 K) |
| Solubility in h2o | 0.3% (twenty°C)[1] |
| Vapor pressure level | 4mmHg or 0.533kPa (20°C)[ane] |
| Magnetic susceptibility (χ) | −89.4·10−6 cm3/mol |
| Refractive alphabetize (northward D) | one.4020 at 20° |
| Hazards | |
| GHS labelling: | |
| Pictograms |   |
| Point give-and-take | Danger |
| Chance statements | H226, H315, H319, H335, H336, H372 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P312, P314, P321, P332+P313, P337+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1 3 0 |
| Flash point | 25 °C (77 °F; 298 Yard) |
| Explosive limits | 1.0% (100°C) – vii.five%[1] |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 7422mg/kg (rabbit, oral) 16,600mg/kg (rat, oral)[2] |
| LCLo (lowest published) | 6470ppm (true cat)[two] |
| NIOSH (The states health exposure limits): | |
| PEL (Permissible) | TWA 100ppm (525mg/grand3)[1] |
| REL (Recommended) | TWA 100ppm (525mg/1000three)[1] |
| IDLH (Immediate danger) | 1000ppm[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Isoamyl acetate, too known as isopentyl acetate, is an organic compound that is the ester formed from isoamyl alcohol and acetic acid. It is a colorless liquid that is only slightly soluble in h2o, but very soluble in most organic solvents. Isoamyl acetate has a strong odor which is described as like to both banana and pear.[3] Pure isoamyl acetate, or mixtures of isoamyl acetate, amyl acetate, and other flavors may be referred to every bit banana oil.[4]
Production [edit]
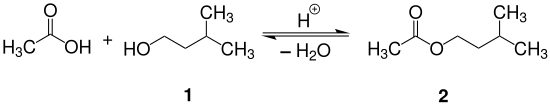
Isoamyl acetate is prepared by the acid catalyzed reaction (Fischer esterification) between isoamyl booze and glacial acerb acid as shown in the reaction equation below. Typically, sulfuric acid is used as the catalyst. Alternatively, p-toluenesulfonic acid or an acidic ion commutation resin can exist used equally the catalyst.
Applications [edit]
Isoamyl acetate is used to confer banana or pear flavor in foods.[v] Banana oil commonly refers to a solution of isoamyl acetate in ethanol that is used as an bogus season.
It is also used as a solvent for some varnishes and nitrocellulose lacquers. As a solvent and carrier for materials such as nitrocellulose, information technology was extensively used in the shipping manufacture for stiffening and air current-proofing fabric flying surfaces, where it and its derivatives were generally known equally 'aircraft dope'. Now that most aircraft wings are made of metal, such use is more often than not limited to historically accurate reproductions and scale models.
Because of its intense, pleasant olfactory property and its depression toxicity, isoamyl acetate is used to test the effectiveness of respirators or gas masks.[vi]
Occurrence in nature [edit]
Isoamyl acetate occurs naturally in the banana plant[seven] and it is also produced synthetically.[8]
Isoamyl acetate is released past a honey bee'southward sting apparatus where information technology serves as a pheromone beacon to attract other bees and provoke them to sting.[ix]
References [edit]
- ^ a b c d e f one thousand NIOSH Pocket Guide to Chemic Hazards. "#0347". National Institute for Occupational Condom and Health (NIOSH).
- ^ a b "Isoamyl acetate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Prophylactic and Health (NIOSH).
- ^ "Iso-amyl acetate". chemicalland21.com.
- ^ Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann'south Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a11_141.
- ^ "Isoamyl acetate". American Chemical Gild . Retrieved 27 October 2022.
- ^ "Fit Testing Procedures (Mandatory). - 1910.134 App A | Occupational Safety and Health Assistants". www.osha.gov . Retrieved 2020-02-04 .
- ^ McGee, Harold (2003). On Food and Cooking. New York: Scribner.
- ^ Isoamyl Acetate Archived 2010-05-28 at the Wayback Machine, Occupational Safety and Health Administration
- ^ Boch R; Shearer DA; Stone BC (September 8, 1962). "Identification of isoamyl acetate as an agile component in the sting pheromone of the love bee". Nature. England: Nature Publishing Group. 195 (4845): 1018–xx. doi:ten.1038/1951018b0. PMID 13870346. S2CID 4224788.
Molar Mass Of Isopentyl Alcohol,
Source: https://en.wikipedia.org/wiki/Isoamyl_acetate
Posted by: carpenterboas1961.blogspot.com



0 Response to "Molar Mass Of Isopentyl Alcohol"
Post a Comment